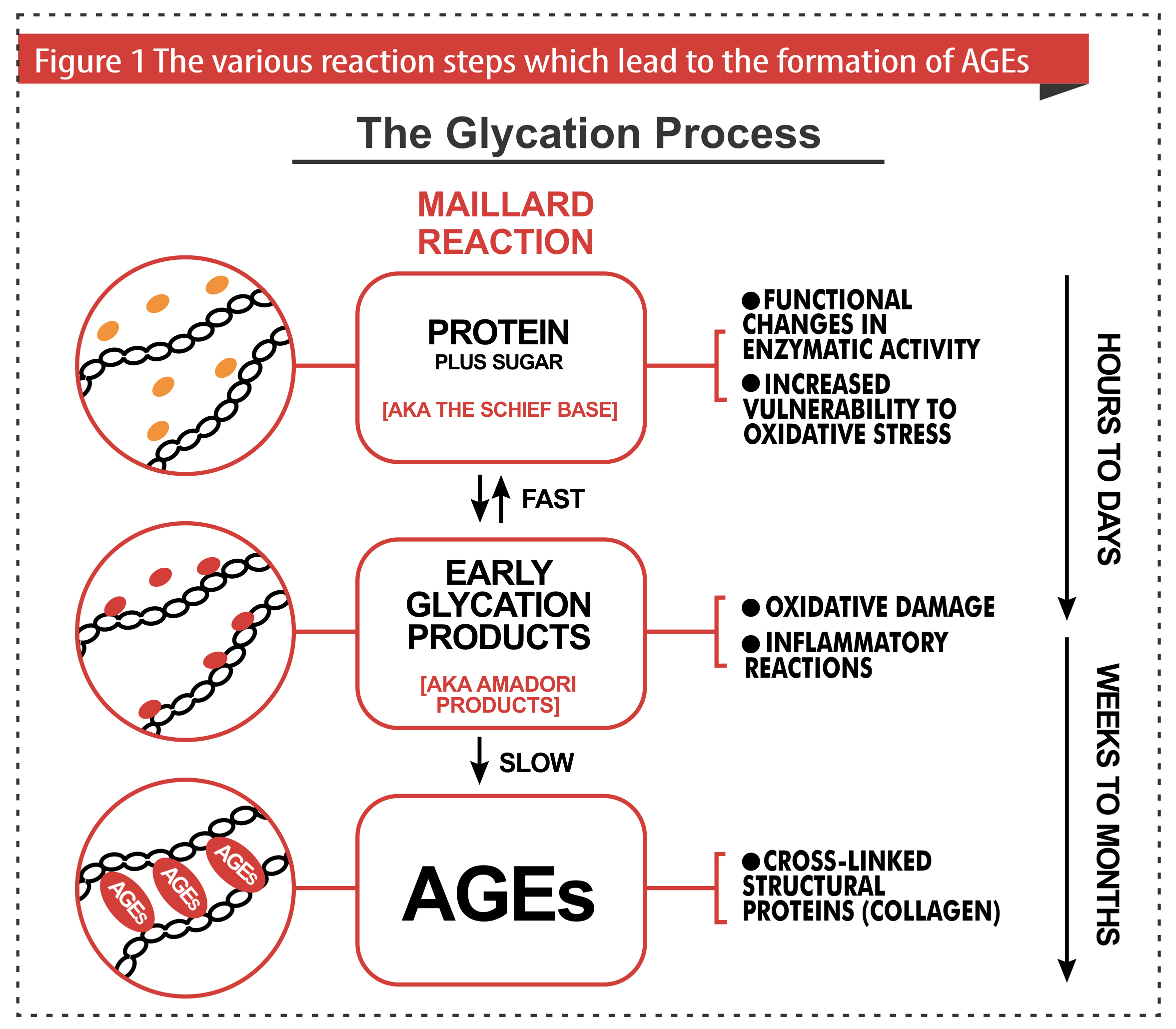
When sugar gets attached to a protein, it forms an unstable Schiff base. (Because it’s unstable, it does not accumulate in the body, but it will rearrange its structure to become an Amadori product or break down the original sugar and protein molecules.) Schiff bases and amadori products are reversible, but only if they do not react with amino, sulfhydryl, and guanidine groups in proteins. If the body can stop the early process of glycation, then it is reversible. If not, it continues in its destructive irreversible pathway, forming protein adducts/lesions and protein crosslinks (as we normally see when sugars attach to proteins such as collagen and elasticity, destroying their integrity and stiffening them).
Your skin isn’t the only place where you will find the damaging effects of glycation. You can find them all over the body, including the brain, heart and eyes. It has been proven that higher blood sugar levels that signal insulin will signal your cells to take in more glucose by way of Glucose Transporter type 4 (GLUT-4). If the body’s insulin doesn’t cooperate with GLUT-4 by allowing the insulin into the cells, the condition that results is called insulin resistance. The common link between cancer, heart disease, Alzheimer’s and depression is too much insulin. It can lead to dangerous excess levels of sugar that are ready to react to any proteins, lipids, and nucleic acids in you skin and body. [3]
Cross-linking is healthy process that takes place as cells move from the basal most bottom layer of the skin to the stratum corneum/epidermis. In order to be flattened structures on surface of skin, cells go through many enzymatic cross-linking processes. [4]
Cross-linking becomes a problem when dangerous sugars are cross-linking to proteins, lipids and nucleic acids vital for the integrity of skin. [4]
Focus on Glycation
The process of glycation occurs when excess glucose attaches to collagen and elastin. Irreversible compounds called AGES (formation of adduct/lesions) accumulate on important proteins all over the body and cause collagen and elastin fibers to be stiffened and less elastic. AGE-modified proteins lose proper functioning and begin to degrade. Skin that has a yellowish and brownish hue is a sign of damaged collagen and elastin from the accumulation of these adducts from glycation.
AGES also cause DNA damage. Glycation can cause your skin to be more photosensitive, resulting in photo-aging stress that can contribute to hyper- pigmentation. The worst thing you can do is to eat or drink something with a lot of sugar and bake in the sun. This increased photosensitivity will weaken the protection of your DNA (see sun damage and pigmentation) and free radicals will be generated by both the glycation and ultraviolet rays, resulting in proteins being destroyed that support the integrity of your skin. [4]
Glycation is the source of problem when someone at high risk in this category suffers from inflammation and free radical damage — and free radicals and inflammation feed each other. Glycation causes damage to membrane lipids and elastin fibers. This will cause the elasticity to be compromised and you’ll have loose skin. When you have excess glucose circulating in the body because of eating food with too much sugar, the glucose will cross-react with (attach to) the chemical bonds between the proteins in the extra cellular matrix and the rest of the body where proteins reside. An example of this occurring in other parts is in the brain, where too much glucose causes elevated HBA1c. When the glucose attaches to iron in the body and brain, it causes free radical damage to lipid membranes of the cells. Cross-linking can happen all over the body.
Glycation also damages the Dermal Epidermal Junction (DEJ), which helps keep the dermis and epidermis working together in harmony for important processes. It is where your dermis connects to your epidermis and is important for cellular communication, nutrient exchange and absorption, and other skin functions. Keratinization of the skin is an integral part of skin renewal and healing. Glycation also promotes MMP secretion (discussed in Firmness and Elasticity).
The Effect of Lifestyle Choices
Even if your risk is not high in this category, you can still promote accelerated glycation by lifestyle choices. Excess sugars in the body will create the glycation cascade. As we age, there is a decline in the enzyme called glyoxylase I, which removes dicarbonyl compounds that create AGES. [4]
Whether you age or consume excess sugars, you’re susceptible to glycation; we all are. Those with high risk have to be extra careful. Anything external that produces AGES and is entered into your body can cause glycation to occur (e.g., baked goods or anything that causes a Maillard reaction). At high heat, the sugars in food react with the proteins.
Also, if you smoke cigarettes, you are inhaling AGES from the tobacco leaves drying in sugar. And another example of glycation caused by external sources is self-tanning products. “The tanning reaction is actually a sophisticated chemical reaction between the sugar in the self tanning cream and the protein found in the Stratum Corneum.” [5]
“Sunless tanning preparations contain dihydroxyacetone (DHA), which undergoes the Maillard reaction with skin protein to produce brown colour.”6 This is the type of reaction you do not want, especially if you have a high risk of glycation or live a lifestyle that promotes excess glucose. Most ingredients will reach dead cells, but will also penetrate deeper to affect living epidermal layers.
Supplements Can Help
You will need supplements that will help with metabolism of glucose and keep your skin and body from accumulating advanced glycated products.
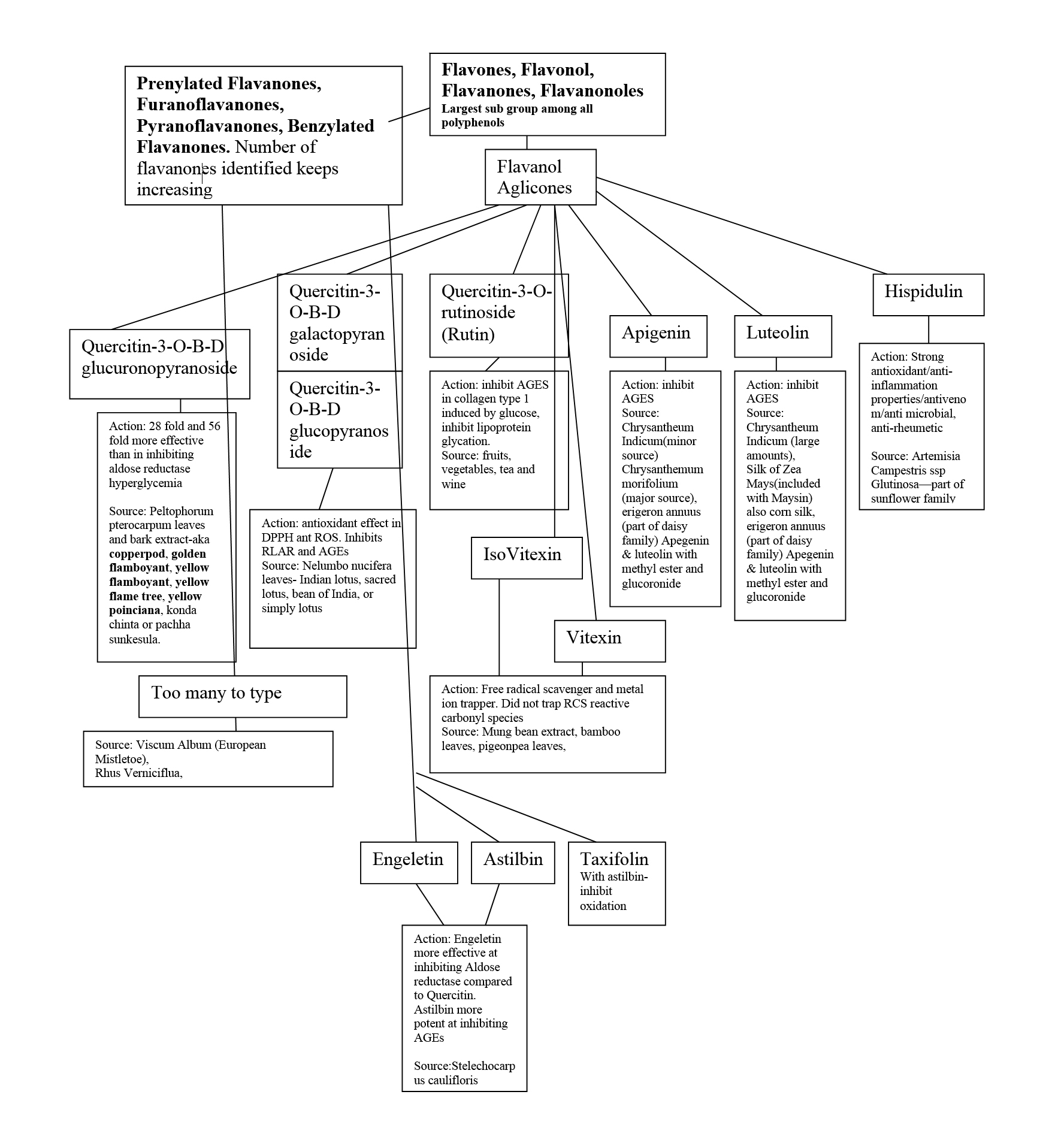
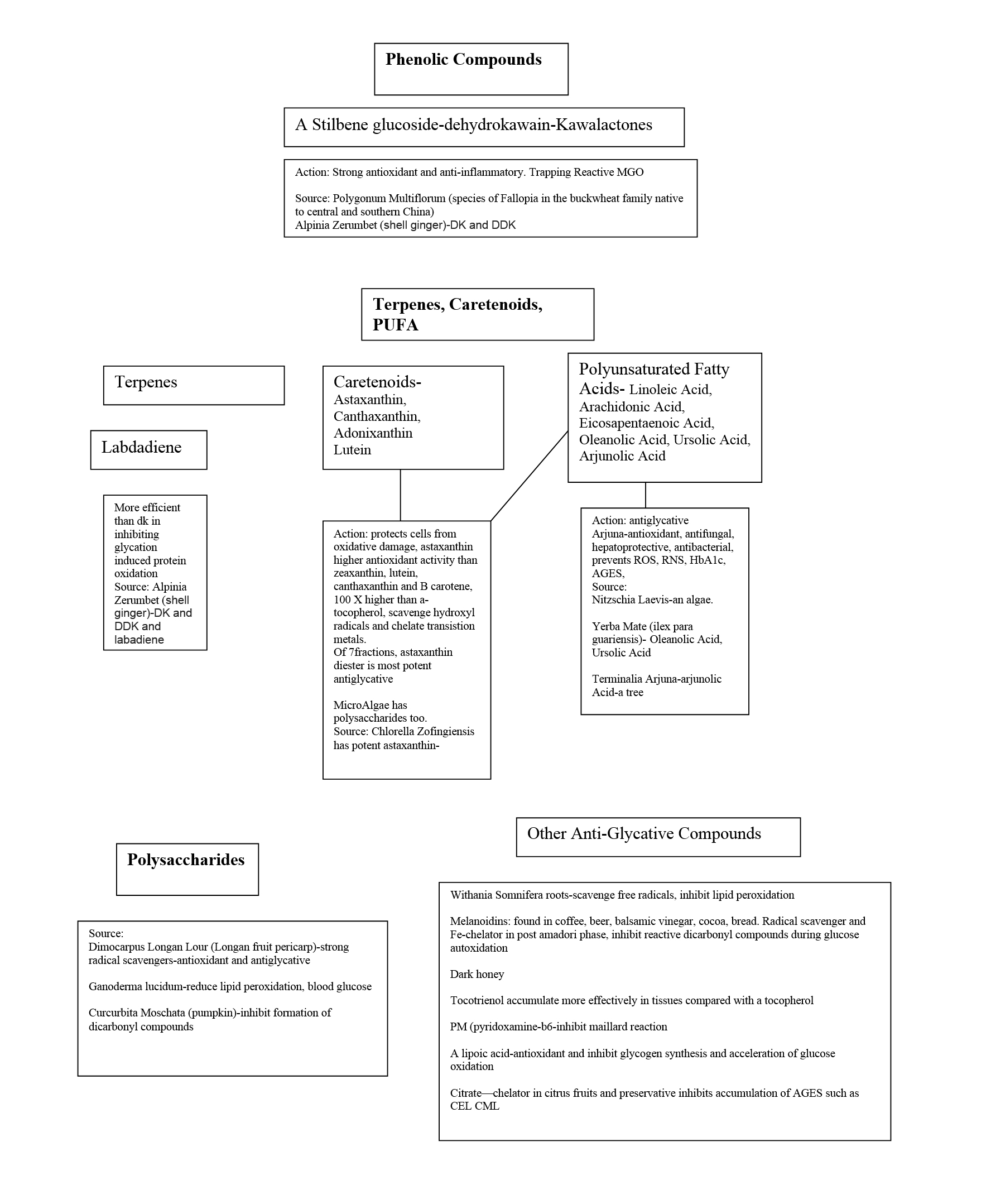
Flavanoids and flavonoid-related ingredients help tremendously by providing anti-glycation activity.
Thiamin Pyrophosphate (TPP), the active coenzyme form of the B-complex vitamin thiamin. In 1996, researchers showed that TPP could step in to stop AGES formation at the most important point in the process: the late, irreversible conversion of Amadori products into full-blown AGES. [7]
“With advancing age, even normal blood sugar levels, given sufficient time, produce AGES that act on RAGES (receptor sites for AGES) to induce deadly inflammation in blood vessels, nerves, liver, and other vital tissues.” [8]
The activation of the RAGES activates pathways that activate Nuclear Factor Kappa B (NFKB), the cytokine/inflammatory signaler. You want to make sure you take supplements that will help with that pathway. Several articles show the benefits of curcumin suppressing NFKB.
It’s been shown that RAGES are expressed in the proteins of fibroblasts and keratinocytes and the expression was increased in ultraviolet exposed skin.
“RAGE signaling can directly induce oxidative stress by decreasing the activity of superoxide dismutase (SOD) or indirectly by reducing cellular anti-oxidant defenses.” [8]
Oxidation from Reactive Oxygen Species (ROS —free radicals, unstable molecules) induced by ultraviolet and other sources causes more AGES and less of a defense against them. This is important to know if you’re at high risk for the free radical category or sun damage. The cross-linking that takes place as a result of glycation increases MMP’s matrix metalloproteinases. (See Firmness and Elasticity category.)
AGES also disrupt the proliferation of keratinocytes, the process of cell renewal in producing new corneocytes that will help your stratum corneum. It’s important for proper skin barrier functioning and needed to shield from pollutants, etc. (See sensitivity and inflammation category)
Aspirin is found to be helpful for glycation and is an anti-inflammatory. Saloxacin is a safer alternative and helps with this.
Glycation can also cause lipid peroxidation. This is when cellular lipids become oxidized by the free radicals generated by glycation. Flavanoids and polyphenols are very helpful with neutralizing lipid peroxidation and glycation. [9]
Figure 4 is a diagram created from the information in the article, “Plant-Derived Agents with Anti-Glycation Activity,” to show the substances that are helpful with preventing glycation.